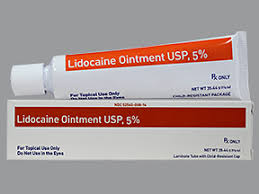
Strides Pharma Science announced that its step‐down wholly owned subsidiary, Strides Pharma Global, Singapore, has received approval for Lidocaine Ointment USP 5% from the United States Food & DrugAdministration (US FDA). Lidocaine Ointment is a generic version of Xylocaine® Ointment of AstraZeneca Pharmaceuticals LP.
According to IQVIA MAT data, the US market for Lidocaine Ointment USP 5% is approximately US$ 50 Mn. The product will be marketed by Strides Pharma Inc. in the US Market and will be launched immediately.
0 thoughts on “Strides receives USFDA approval for Lidocaine Ointment”