Even then, Lupin spends about 10% of its revenue on research and development, which tends to aid in future product filings. Lupin has 40 first-to-file (FTF) filings including 14 exclusive FTF opportunities. However, some of the benefits accruing from this product pipeline could be seen in FY21.
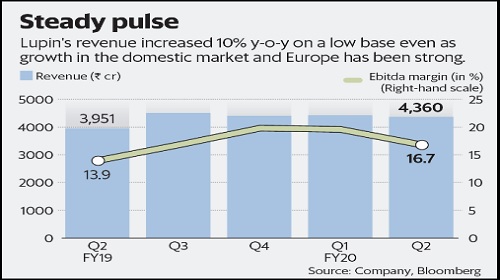
The India business has been stable, though sequential revenue growth is marginal at about 2.6%. The European and Middle East businesses have had a standout quarter delivering a growth rate of about 23% quarter-on-quarter (q-o-q). Still, the lower operating metrics overall meant that operating margins contracted q-o-q.
This quarter, though, a lawsuit settlement in theTexas, US, has seen the company report a loss of ₹123 crore. Lupin had to make a provision of ₹379 crore on account of the lawsuit.
Additionally, Lupin also incurred a loss of ₹167 crore on sale of its stake in Kyowa Criticare Co, Japan. Adjusted for all this, net profit would have been higher.
Still, progress in the US market with regard to new launches, and the pace of FDA resolution will be a key variable to watch. The stock trades at a price-to-earnings multiple of about 22 times FY21 earnings per share.
" />
Lupin Ltd’s second quarter earnings were marginally lower than what the market had anticipated. The stock perked up a bit on the results, but ended Wednesday on a flat note.
Lupin’s US business has contracted marginally after one of its drugs came out of the exclusive list. Besides, price pressure in the US market continues which also reduced sales. Hence, US revenue fell by about 15% in Q2 FY20 from the first quarter. Analysts consider the US market as challenging going ahead.
Besides, much depends on how the new launches in the US pan out. In the coming quarters, Lupin has set a target of about 25-30 product launches. But the company also has to settle the USFDA issues and observations regarding its plants quickly. The management clarified in a post-earnings conference call that it is looking at a re-inspection of some plants sometime early in 2020. However, product filings from these plants may be slow until the issue is resolved.
Even then, Lupin spends about 10% of its revenue on research and development, which tends to aid in future product filings. Lupin has 40 first-to-file (FTF) filings including 14 exclusive FTF opportunities. However, some of the benefits accruing from this product pipeline could be seen in FY21.
The India business has been stable, though sequential revenue growth is marginal at about 2.6%. The European and Middle East businesses have had a standout quarter delivering a growth rate of about 23% quarter-on-quarter (q-o-q). Still, the lower operating metrics overall meant that operating margins contracted q-o-q.
This quarter, though, a lawsuit settlement in theTexas, US, has seen the company report a loss of ₹123 crore. Lupin had to make a provision of ₹379 crore on account of the lawsuit.
Additionally, Lupin also incurred a loss of ₹167 crore on sale of its stake in Kyowa Criticare Co, Japan. Adjusted for all this, net profit would have been higher.
Still, progress in the US market with regard to new launches, and the pace of FDA resolution will be a key variable to watch. The stock trades at a price-to-earnings multiple of about 22 times FY21 earnings per share.
0 thoughts on “At Lupin, all eyes on progress of new launches pace of FDA resolution”